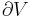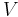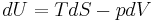Laws of thermodynamics
| Thermodynamics | |||||||||||||||||||||||||||||||||||||||||||||||

|
|||||||||||||||||||||||||||||||||||||||||||||||
The laws of thermodynamics describe the transport of heat and work in thermodynamic processes. These laws have become some of the most important fundamental laws in physics and other sciences associated with thermodynamics.
Classical thermodynamics, which is focused on systems in thermodynamic equilibrium, can be considered separately from non-equilibrium thermodynamics. This article focuses on classical or thermodynamic equilibrium thermodynamics.
The four principles (referred to as "laws"):[1][2][3][4][5][6]
- The zeroth law of thermodynamics, which underlies the basic definition of temperature.
- The first law of thermodynamics, which mandates conservation of energy, and states in particular that the flow of heat is a form of energy transfer.
- The second law of thermodynamics, which states that the entropy of an isolated macroscopic system never decreases, or (equivalently) that perpetual motion machines are impossible.
- The third law of thermodynamics, which concerns the entropy of a perfect crystal at absolute zero temperature, and which implies that it is impossible to cool a system all the way to exactly absolute zero.
There have been suggestions of additional laws,[7] but none of them have anything like the generality of the accepted laws, and they are not mentioned in standard textbooks.[1][2][3][4][8][9][5]
Contents |
Zeroth law
If two thermodynamic systems are each in thermal equilibrium with a third, then they are in thermal equilibrium with each other.
When two systems, each in its own thermodynamic equilibrium, are put in purely thermal connection, radiative or material, with each other, there will be a net exchange of heat between them unless or until they are in thermal equilibrium. That is the state of having equal temperature. Although this concept of thermodynamics is fundamental, the need to state it explicitly was not widely perceived until the first third of the 20th century, long after the first three principles were already widely in use. Hence it was numbered zero -- before the subsequent three. The Zeroth Law implies that thermal equilibrium, viewed as a binary relation, is a transitive relation. Since a system in thermodynamic equilibrium is defined to be in thermal equilibrium with itself, and, if a system is in thermal equilibrium with another, the latter is in thermal equilibrium with the former. Thermal equilibrium is furthermore an equivalence relation.
First law
Energy can be neither created nor destroyed. It can only change forms.
In any process in an isolated system, the total energy remains the same.
For a thermodynamic cycle the net heat supplied to the system equals the net work done by the system.
The First Law states that energy cannot be created or destroyed; rather, the amount of energy lost in a steady state process cannot be greater than the amount of energy gained. This is the statement of conservation of energy for a thermodynamic system. It refers to the two ways that a closed system transfers energy to and from its surroundings – by the process of heat transfer and the process of mechanical work. The rate of gain or loss in the stored energy of a system is determined by the rates of these two processes. In open systems, the flow of matter is another energy transfer mechanism, and extra terms must be included in the expression of the first law.
The First Law clarifies the nature of energy. It is a stored quantity which is independent of any particular process path, i.e., it is independent of the system history. If a system undergoes a thermodynamic cycle, whether it becomes warmer, cooler, larger, or smaller, then it will have the same amount of energy each time it returns to a particular state. Mathematically speaking, energy is a state function and infinitesimal changes in the energy are exact differentials.
All laws of thermodynamics but the First are statistical and simply describe the tendencies of macroscopic systems. For microscopic systems with few particles, the variations in the parameters become larger than the parameters themselves, and the assumptions of thermodynamics become meaningless.
Fundamental thermodynamic relation
The first law can be expressed as the fundamental thermodynamic relation:
Heat supplied to a system = increase in internal energy of the system + work done by the system
Increase in internal energy of a system = heat supplied to the system - work done by the system
Where:
- U is internal energy
- T is temperature
- S is entropy
- p is pressure
- V is volume
This is a statement of conservation of energy: The net change in internal energy (dU) equals the heat energy that flows in (TdS), minus the energy that flows out via the system performing work (pdV).
Second law
Consider two isolated systems in separate but nearby regions of space, each in thermodynamic equilibrium in itself (but not in equilibrium with each other). Then let some event break the isolation that separates the two systems, so that they become able to exchange matter or energy. Wait till the exchanging systems reach mutual thermodynamic equilibrium. Then the sum of the entropies of the initial two isolated systems is less than or equal to the entropy of the final exchanging systems. In the process of reaching a new thermodynamic equilibrium, entropy has increased (or at least has not decreased). Both matter and energy exchanges can contribute to the entropy increase.
In a few words, the second law states "spontaneous natural processes increase entropy overall." Another brief statement is "heat can spontaneously flow from a higher-temperature region to a lower-temperature region, but not the other way around." Nevertheless, energy can be transferred from cold to hot, for example, when a refrigerator cools its contents while warming the surrounding air, though still all transfers as heat are from hot to cold. Heat flows from the cold refrigerator air to the even-colder refrigerant, then the refrigerant is warmed by compression (which requires an external source of energy to do thermodynamic work), then heat flows from the hot refrigerant to the outside air, then the refrigerant cools by expansion to its initial volume (thus doing thermodynamic work on the environment), and the cycle repeats. Entropy is increased also by processes of mixing without transfer of energy as heat.
A way of thinking about the second law is to consider entropy as a measure of ignorance of the microscopic details of the motion and configuration of the system given only predictable reproducibility of bulk or macroscopic behaviour. So, for example, one has less knowledge about the separate fragments of a broken cup than about an intact one, because when the fragments are separated, one does not know exactly whether they will fit together again, or whether perhaps there is a missing shard. Solid crystals, the most regularly structured form of matter, with considerable predictability of microscopic configuration, as well as predictability of bulk behaviour, have low entropy values; and gases, which behave predictably in bulk even when their microcopic motions are unknown, have high entropy values. This is because the positions of the crystal atoms are more predictable than are those of the gas atoms, for a given degree of bulk predictability.
The entropy of an isolated macroscopic system never decreases. However, a microscopic system may exhibit fluctuations of entropy opposite to that stated by the Second Law (see Maxwell's demon and Fluctuation Theorem).
Third law
As temperature approaches absolute zero, the entropy of a system approaches a constant minimum.
Briefly, this postulates that entropy is temperature dependent and results in the formulation of the idea of absolute zero.
History
Although it is customary for physicists to ridicule Aristotle, it is fair[10][11][12][13] to say that he seems perhaps to have helped science consider the concept of an underlying constitutive undetermined entity that appears in different forms as natural processes evolve but is not itself changed, and fair to say that energy is the present-day notion that fits Aristotle's conception. To Aristotle's conceptual genius we can perhaps attribute the scientific articulation of the distinction between the form, μορφή, which changes, and the underlying constitutive undetermined entity, ὕλη, which does not change, in the course of natural process. The distinction between ὕλη and μορφή came to Aristotle partly from Plato who wrote of 'the receptacle', δοχή, and 'the idea', εἶδος[14][15].
In the modern era, the historically first established thermodynamic principle which eventually became the Second Law was formulated by Sadi Carnot during 1824. By 1860, as formalized in the works of those such as Rudolf Clausius and William Thomson, there were two established "principles" of thermodynamics, the first principle and the second principle. As the years passed, these principles were termed "laws." By 1873, for example, thermodynamicist Josiah Willard Gibbs, in his “Graphical Methods in the Thermodynamics of Fluids”, clearly stated that there were two absolute laws of thermodynamics, a first law and a second law. Some textbooks throughout the 20th century have also numbered the laws slightly differently. In some fields removed from chemistry, the second law was considered to deal with the efficiency of heat engines only, whereas what was called the third law dealt with entropy increases. (And directly defining zero points for entropy calculations was not considered to be a law.) Gradually the older second and third laws have been combined into the second law and the more modern third law has become widely adopted.
See also
- Conservation law
- Heat death of the universe
- Laws of science
- Philosophy of thermal and statistical physics
- Table of thermodynamic equations
References
- ↑ 1.0 1.1 Guggenheim, E.A. (1985). Thermodynamics. An Advanced Treatment for Chemists and Physicists, seventh edition, North Holland, Amsterdam, ISBN 0444869514.
- ↑ 2.0 2.1 Kittel, C. Kroemer, H. (1980). Thermal Physics, second edition, W.H. Freeman, San Francisco, ISBN 0716710889.
- ↑ 3.0 3.1 Adkins, C.J. (1968). Equilibrium Thermodynamics, McGraw-Hill, London, ISBN 0070840571.
- ↑ 4.0 4.1 Kondepudi D. (2008). Introduction to Modern Thermodynamics, Wiley, Chichester, ISBN 9780470015988.
- ↑ 5.0 5.1 Lebon, G., Jou, D., Casas-Vázquez, J. (2008). Understanding Non-equilibrium Thermodynamics. Foundations, Applications, Frontiers, Springer, Berlin, ISBN 9783540742524.
- ↑ Chris Vuille; Serway, Raymond A.; Faughn, Jerry S. (2009). College physics. Belmont, CA: Brooks/Cole, Cengage Learning. p. 355. ISBN 0-495-38693-6. http://books.google.ca/books?id=CX0u0mIOZ44C&pg=PT355.
- ↑ For example: Honig suggests an upper limit to temperature: [1]; Jørgensen suggests that ecosystems will take advantage of exergy sources [2]
- ↑ De Groot, S.R., Mazur, P. (1962). Non-equilibrium Thermodynamics, North Holland, Amsterdam.
- ↑ Glansdorff, P., Prigogine, I. (1971). Thermodynamic Theory of Structure, Stability and Fluctuations, Wiley-Interscience, London, ISBN 0471302805.
- ↑ Aristotle, Physics ii 2 193a 28-30.
- ↑ Aristotle, Metaphysics vii 3 1029a 2.
- ↑ Graham, D.W. (1987). Aristotle's Two Systems, Clarendon Press, Oxford UK, ISBN0198249705, section 3.1.1 .
- ↑ Lang, H.S. (1998). The Order of Nature in Aristotle's Physics: Place and the Elements, Cambridge University Press, Cambridge UK, ISBN 978052164534, page 52.
- ↑ Plato, Timaeus, 50D-51B5.
- ↑ Lang, H.S. (1998). The Order of Nature in Aristotle's Physics: Place and the Elements, Cambridge University Press, Cambridge UK, ISBN 978052164534, page 50.
See also Conversation of heat
Further reading
- Goldstein, Martin, and Inge F., 1993. The Refrigerator and the Universe. Harvard Univ. Press. A gentle introduction.